A point of principle
24 June 2021Novelty is one of the three mandatory criteria for patentability of an invention established by the Patent Law of the Russian Federation, the Eurasian patent legislation, and also by the patent legislation of all countries where there is a patent protection system.
Moreover, the “novelty” patentability criterion of an invention has always been considered as the most clearly defined and simple enough to establish that a claimed invention complies with it.
However, the practice of consideration of applications for inventions and administrative cases on invalidation of Russian and Eurasian patents by the Federal Service for IP (Rospatent) in recent years and the consideration of such cases by the IP Court shows that the long-standing approaches to evaluating the “novelty” patentability criterion of an invention have been notably changing.
At the same time, there is an inexplicable trend to pivot from the principles of evaluating this criterion adopted worldwide to those previously applied as an exception to the general rule in case of extremely specific and limited situations.
To understand what basic requirements are established by the current patent legislation of the Russian Federation and the Eurasian Patent Convention for the “novelty” patentability criterion of an invention, a brief overview of the relevant provisions of the Russian and Eurasian patent legislations is provided below.
The key condition for compliance of an invention with the “novelty” patentability criterion both in accordance with the Civil Code of the Russian Federation (chapter 4, article 1350, clause 4) (CC RF) and in accordance with the Instruction to the Eurasian Patent Convention (rule 3 [1]) (the Instruction to the EAPC) is as follows.
An invention is new if it is unknown in the art/is not a part of the prior art
The “novelty” patentability criterion of an invention is defined in more detail in the “Rules for Preparation, Filing, and Consideration of Documents Constituting a Basis for Taking Legal Actions on State Registration of Inventions and Their Forms” (RU PTO Rules) and the “Rules for Preparation, Filing, and Consideration of Eurasian Applications with the Eurasian Patent Office” (EAPO Rules).
In accordance with the RU PTO Rules (clause 70): “During novelty assessment, an invention is recognised as new if it is established that the set of features of the invention presented in an independent claim of the set claims is unknown from the information that has become publicly available worldwide before the priority date of the invention.”
The RU PTO Administrative Regulations adopted in 2008 and (currently repealed) contained a slightly different definition of novelty of an invention: “An invention is recognised as known in the prior art and not compliant with the novelty criterion if the prior art discloses a means having all features inherent to the invention characterised by the set of claims proposed by the applicant.”
In accordance with the EAPO Rules (clause 5.7): “The check for novelty is conducted for the entire set of the features characterising the invention, ie, contained in the set of the claims. The invention is not recognised as new if the prior art reveals information about a subject that has features identical to all features contained in an independent claim of the set of claims.”
Thus, both Russian and Eurasian patent legislations clearly establish that it is necessary to identify in the prior art a mean/subject characterised by the set of features identical to those characterising the invention in the set of claims.
At the same time, in accordance with the “Explanatory Dictionary of the Russian Language” (SI Ozhegov, N Yu Shvedova): “Identical means the same, being entirely identical.”
Based on the above requirements, it is obvious that an invention may be recognised as non-compliant with the “novelty” patentability criterion only if a mean/subject characterised by an entirely identical set of features is disclosed in the prior art.
In terms of the generally accepted interpretation of the meaning of the term “identical”, it can by no means be concluded that the feature expressed by the general concept (and a range of any numerical values is undoubtedly a feature expressed by the general concept) is identical to (or entirely congruent with) the feature expressed by a particular or narrower concept (in particular by the narrower range).
It follows from the above analysis that the Russian and Eurasian legislation provides for no exception to the established general approach to evaluation of the “novelty” patentability criterion.
This conclusion is also supported by the explanations provided in the RU PTO Guidelines adopted in 2011, which is now repealed (clause 5.4.2), and the current RU PTO Guidelines adopted in 2018 (clause 2.9.10), which clearly define the general approach to assessment of the “novelty” patentability criterion, which is that “a general disclosure does not usually deprive a particular disclosure of novelty but a particular disclosure deprives the general claims, covering the particular disclosure, of novelty”.
The guidelines of 2011 and 2018 are not legislation but they are recommendations to the RU PTO examiners on the use of certain methodological approaches developed by the practice of application of the relevant provisions of the Russian legislation.
Furthermore, these guidelines contain no general provisions establishing that any invention based on the “narrower–broader” principle should be recognised as non-compliant with the “novelty” patentability criterion.
The concept of “an invention directed to the known solution based on the ‘narrower–broader’ principle” is contained only in the guidelines of 2011 and refers to very specific cases, which examples are given in clause 13 of the guidelines as an exception to the general rule for evaluation of the “novelty” patentability criterion specified in clause 5.4.2 of this document. The later guidelines of 2018 do not even define an invention correlated with the known solution based on the “narrower–broader” principle.
When discussing novelty of the inventions correlating with the prior art solutions as “narrower–broader”, Guidelines 2011 provides examples of the inventions that the recommended approach refers to. From careful study of the mentioned examples it becomes obvious that the specific approach given in Guidelines 2011 concerns only inventions consisting of several specific components amounts, which are characterised by some value ranges completely covered by the more broad value ranges of the same components in the composition known from the prior art .
The approach to evaluation of invention novelty based on the “narrower–broader” principle described in the guidelines of 2011 is not even expected to apply to any other cases.
The current guidelines of 2018 do not contain any exceptions to the above general approach to assessment of the “novelty” patentability criterion of an invention, in particular it does not mention the “narrower–broader” approach even to specific cases.
The EAPO approach to novelty assessment
The Eurasian patent legislation contains no special requirements for assessment of novelty of inventions that correlate with the known solution based on the “narrower–broader” principle.
However, the EAPO has developed and applied the following approach to novelty assessment of such inventions.
The key condition for recognition of novelty is that the invention characterised by the selected parameter(s) or more narrow value(s) range is new only if this particular parameter(s) or narrow value(s) range is/are not explicitly disclosed for the same means in the prior art.
The invention characterised by the selected parameter or values range may be recognised as new provided that the following three conditions are simultaneously fulfilled:
a) the selected parameter of values range is narrow compared to the known range of values;
b) the selected parameter of values range is sufficiently distant from the values disclosed particularly within the broad range and from the end values of the known broad range; and
c) in the selected range, a technical result different from the one known for the broad range, ie, a new unexpected technical result, should be achieved.
As well, the current Russian and Eurasian patent legislations provide for no exceptions to the general principle for evaluation of the “novelty” patentability criterion of an invention for so-called “selective inventions”—a chemical compound covered by the general structural formula of a group of known compounds—because a group of compounds characterised by the general structural formula does not disclose any individual structure of each compound.
Therefore, the general formula of a group of compounds is not characterised by a set of features identical to an individual structure of the particular chemical compound.
Moreover, when assessing the novelty of an individual chemical compound, the general principle is that “a general disclosure does not anticipate novelty of a particular disclosure”.
This principle for assessment of novelty of a chemical compound covered by the general structural formula of a group of known compounds was first introduced in clause 24.5.2 of the RU PTO Administrative Regulations 2008 and is now envisaged in clause 70 of the RU PTO Rules.
The EAPO applies the same approach to evaluation of the “novelty” patentability criterion of the said invention.
There are no exceptions to the generally accepted approach to evaluation of the “novelty” patentability criterion for inventions relating to a new use of known means, devices, or methods, since, taking into consideration the fact that the only distinctive feature of these inventions is their new purpose, this feature is always taken into consideration when evaluating whether a set of features for a means, item, or method known in the art is identical.
Based on the above analysis of the provisions of the current Russian and Eurasian legislations, it may be concluded that two key principles are applied to evaluate whether an invention complies with the “novelty” patentability criterion:
- a set of features characterising the means/subject in the art is identical to the set of features characterising the invention in the claims of the invention; and
- a general disclosure does not usually anticipate novelty of a particular disclosure, but a particular disclosure anticipates novelty of the general concept, covering the particular disclosure.
Neither Russian nor Eurasian legislation establishes any other approaches to assessment of this patentability criterion.
It is in connection with the above that the current trends in evaluation of the “novelty” patentability criterion of an invention, clearly observed in the decisions of the RU PTO and the IP Court and consisting in use of the “mechanic” approach of the “narrower–broader” principle not only in the specific cases considered in the Guidelines 2011 but also to any cases related to inventions characterised by a combination of features correlated with the features of a means/subject known in the art based on the “narrower–broader” principle, are particularly surprising and quite alarming.
Moreover, this principle applies not only to the quantitative features characterising the invention and expressed as values ranges, but also to the qualitative features characterising the components of the product or the device, correlated as the particular and the general with the characteristics of the means/subject of the prior art, and to the quantitative features characterising the effective amount or the dose of a biologically active substance in the inventions relating to products of a certain therapeutic use.
Furthermore, the “narrower–broader” principle is in some cases applied even for inventions characterised by two and more features, which all correlate with the characteristics of the means subject of the prior art as “the particular–the general”.
At the same time, when considering such inventions, the fact that it follows from the description of the general technical solution known in the art that, when creating this technical solution, no task to create the claimed invention with the advantages and/or new properties it possesses has been set is often not even taken into account.
Case study
Examples illustrating such an approach can be seen in decisions issued by the RU PTO, the IP Court, and the Resolution of the Presidium of the IP Court on Eurasian patent No. 005416.
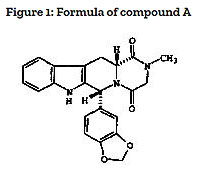 Eurasian patent No. 005416 was issued for the invention characterised in the granted claims of as follows: “Pharmaceutical unit dosage form suitable for oral administration, containing from about 1mg to about 20mg (up to a maximum dose of 20mg a day) of a compound having the structural formula Figure 1 (compound A)”.
Eurasian patent No. 005416 was issued for the invention characterised in the granted claims of as follows: “Pharmaceutical unit dosage form suitable for oral administration, containing from about 1mg to about 20mg (up to a maximum dose of 20mg a day) of a compound having the structural formula Figure 1 (compound A)”.
At the same time, the patent description discloses that, when using this invention, the technical result consisting in maintaining the level of therapeutic effect is achieved by using a substantially lower dose of this active substance with substantially reduced side-effects.
The prior art document (application WO 97/03675) considered as the prior art for “novelty” assessment disclosed a group of compounds characterised by the general structural formula. The list of preferred compounds also specified compound A.
An oral dose from 0.5 to 800mg a day was specified for the group of compounds.
It was also specified that the tablets or capsules contained from 0.2 to 400mg of the active compound in a suitable pharmaceutically acceptable diluent or carrier for administration as one or more doses once or more times a day.
The examples given in the prior art document disclosed capsules and tablets containing 50mg of the active substance for administration once or more often a day.
Thus, it was obvious that the invention under the patent has been created by selecting individual compound A out from the group of compounds, selecting a narrow range of the amount of the active compound in dosage form, and selecting a low maximum daily dose of the active compound.
Therefore, the invention was created by selecting single or narrow values of features out of substantially broader values of features of the known technical solution.
At the same time, the prior art document did not disclose as any subject that has features identical to all features contained in an independent claim of the set of claims, since none of the features characterising the invention under the patent was identical to the relevant feature of the known subject.
The RU PTO made the decision to invalidate the Eurasian patent in the Russian Federation for non-compliance of the patented invention with the “novelty” patentability criterion.
By the IP Court decision of July 8, 2020, the RU PTO decision was kept in force. As the basis of the IP Court decision, the following conclusions were made: “…the dose of 1 to 20mg of tadalafil used in the disputed patent as an active ingredient for treatment of sexual dysfunction is both part of the dose of 0.2 to 400mg and part of the dose of 0.5 to 800mg of tadalafil also disclosed in the document [1] and the methods of administration of the said preparation both in the contested patent and in the document [1] are identical, the IP Court has no grounds to disagree with the above conclusion of Rospatent that the group of inventions under the disputed patent is part of the solution known from the prior art [1], which means that the solution under the disputed patent does not comply with the ‘novelty’ patentability criterion, since all features of the group of inventions under disputed patent EA No. 005416 are known from publication WO 97/03675 [1].”
The Resolution of the IP Court Presidium
The Resolution of the IP Court Presidium, in accordance with which the IP Court decision as of a court of the first instance is kept in force, is even more interesting from the point of view of the use approach to evaluation of novelty. The Resolution of the IP Court Presidium dated July 8, 2020, specifies: “...properties and technical result are a consequence of the features of the invention and not these features themselves and, if the invention does not provide for changes in the known subject (has identical features) but differs from it only in properties or a technical result, it should be recognised as not compliant with the ‘novelty’ patentability criterion.
“If the known physical subject has not changed, then the patent for the invention for this subject as such cannot be issued, since the invention characterising this subject does not comply with the ‘novelty’ patentability criterion.”
Therefore, the analysis of this case clearly shows the “mechanic” use of “narrower–broader” approach in this case despite the fact that neither the features characterising the active substance in the invention the contested patent and in the known subject are identical, since they correlate as “the particular (compound A)–the general (group of compounds)” nor the features characterising the quantity of the active substance are identical, since they correlate as narrower and broader quantitative ranges nor the features characterising the daily dose of the substance are identical, since they also correlate as narrow and broad quantitative ranges.
At the same time, neither the RU PTO nor the IP Court have taken into consideration the fact that the technical solution disclosed in the prior art document clearly relates to production of a group of new biologically active compounds, for which a possible amount in the dosage form and a possible effective daily dose was just approximately determined. The maximum effective dose of the active compound confirmed experimentally was 50mg. At the same time, it is obvious to person skilled in the art that not all compounds from the group could be effective within the entire range of these doses.
The content of the prior art document clearly indicates that no task to create a specific dosage form having sufficient therapeutic effect at the low amount of the active substance and at the low daily dose has been set when creating the technical solution described therein.
This example fully illustrates the unreasonable use of the “narrower–broader” approach in a situation where this approach should not and cannot be applied in accordance with the requirements established for evaluation of the “novelty” patentability criterion by the Eurasian patent legislation.
Moreover, use of the “narrower–broader” approach to evaluate novelty of the invention in this case, among other things, goes beyond the original meaning laid down in this approach only for the exceptional specific cases illustrated in the RU PTO Guidelines 2011 (the only document that is not a legislation, which defines such an approach).
The lack of a unified approach to assessment of the novelty of the invention is clear from the decisions made by RU PTO and IP Court and from the Resolution of the IP Court Presidium rendered with regard to patent of the Russian Federation No. 2488999.
Patent of the Russian Federation No. 2488999 was granted for the invention characterised as follows: “Herbicidal composition comprising, as active substances, effective combination of N-(2,6-difluorophenyl)-8-fluoro-5-methoxy[1,2,4]triazolo[1,5-c]pyrimidine-2-sulfonamide (florasulam) (I) or a salt thereof and another herbicide selected from sulfonylureas characterised in that another herbicide selected from sulfonylureas is 2-[[[[(4-methoxy-6-methyl-1,3,5-triazin-2-yl)methylamino]carbonyl]amino]sulfonyl]benzoic acid methyl ester (tribenuron-methyl) (II) or a salt thereof, while the weight ratio of components I:II ranges from 1:5 to 5:1, and a solid carrier or liquid diluent and a surface active compound.”
The technical result claimed in the contested patent for the said invention was a synergistic effect when the said active substances were used in the said ratio.
The prior art document (patent application CN102037986 [1]) disclosed a herbicidal composition containing (florasulam) (I) and another herbicide (tribenuron-methyl) (II) with a weight ratio of components I: II ranging from 40:1 to 1:20 and a solid carrier or liquid diluent and a surfactant.
Paragraph [0027] of the document [1] specified that the used composition has a “synergistic effect”.
There are examples confirming that the composition is effective for weed control, ie, an “effective combination” is used.
By its decision, the RU PTO invalidated the said patent due to non-compliance of the invention characterised in claim 1 of the set of claims with the “novelty” patentability criterion.
As the grounds for the decision taken, Rospatent stated the following: “...the range of ratios I:II according to the disputed patent, which is from 1:5 to 5:1, entirely falls within the range disclosed in the patent document [1], which is from 40:1 to 1:20.
“The patent document [1] provides examples showing that the known composition is effective for weed control, ie, an ‘effective combination’ is used.
“Since the patent document [1] discloses a herbicidal composition having all features of the composition according to independent claim 1 of the disputed patent and the features of dependent claims 2 to 3 and 4 to 6 (in part) … the known composition according to claims 1 to 3 and 4 to 6 (in part)... does not comply with the ‘novelty’ patentability criterion.”
By the IP Court decision dated October 22, 2018, the RU PTO decision was upheld on the following grounds: “Rospatent has reasonably concluded that the prior art source [1] discloses a herbicidal composition having all features of the composition according to independent claim 1 of the disputed patent (in part of alternative 1) and the features of dependent claims 2 to 3 and 4 to 6 (in part) ... of patent of the Russian Federation for invention No. 2488999.”
However, this IP Court decision has been reversed by the Resolution of the IP Court Presidium dated February 11, 2019, and the case has been remanded for new consideration to the IP Court as a court of first instance.
By the IP Court decision dated October 7, 2019, the RU PTO decision was reversed on the following grounds: “Rospatent has unreasonably concluded that the prior art source discloses a herbicidal composition having all features of the composition according to independent claim 1 of the disputed patent (in part of alternative 1) and the features of dependent claims 2 to 3 and 4 to 6 (in part).
“The court agrees with the applicant’s argument that document No. CH102037986A does not disclose the essential feature of the disputed invention ‘the weight ratio of components (herbicides) I:II can vary from 1:5 to 5:1’.
“The conclusion of Rospatent that the composition according to independent claim 1 ... of the disputed patent does not comply with the ‘novelty’ patentability criterion is unlawful.”
By the Resolution of the IP Court Presidium dated February 10, 2020, the IP Court decision was upheld.
Analysis
An analysis of the above case shows the following. During consideration of an appeal against the patent grant when assessment novelty of the invention, the RU PTO and IP Court as a court of first instance used the “narrower–broader” approach in full accordance with the Guidelines 2011. At the same time, the RU PTO has every reason to use this approach, since the invention has been related to a composition, which qualitative makeup of the components was identical to that of the known composition, while the ratio of the components in the invention has been characterised by the narrow range of quantitative values selected from the broader range of such values of the known composition.
However, in this case, the IP Court Presidium disagreed with lawfulness of such an approach and, during reconsideration, IP Court used the general approach to evaluation of novelty, namely that “a general disclosure does not anticipate novelty of the particular disclosure”. At the same time, the narrower range characterising the invention was recognised as not disclosed in the prior art document even though it was the part of the broader range of values.
It is interesting to analyse the two above cases in comparison (Table 1).
Table 1: Comparison of two patent cases
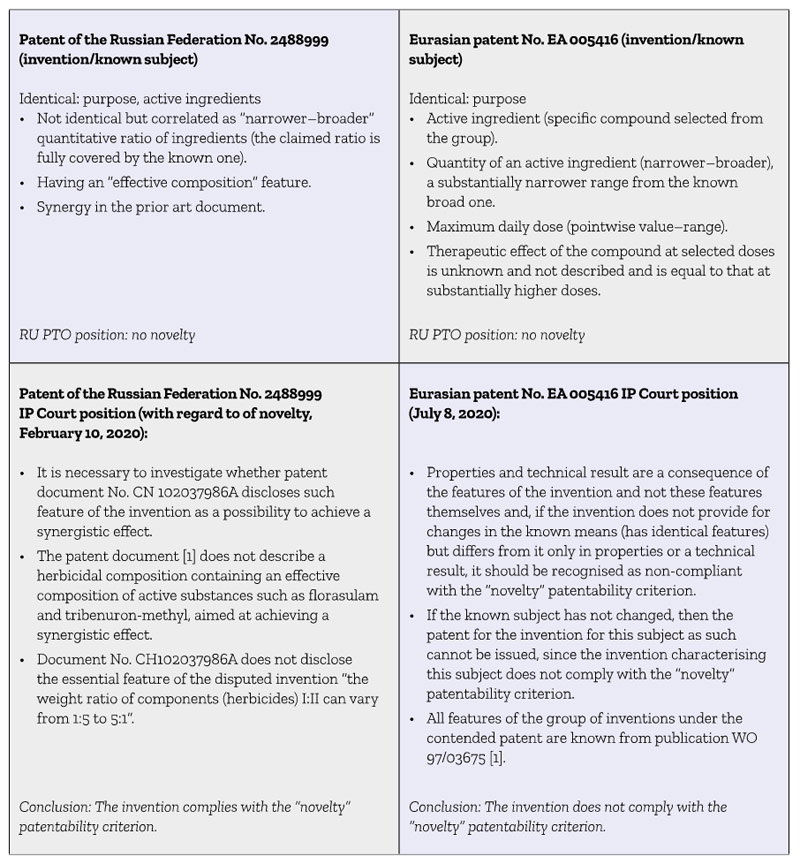
The two above cases fully demonstrate that there are no certain approaches to assessment of the “novelty” patentability criterion in cases where the invention is characterised by the features, part or all of which are correlated with the characteristics of technical solutions of the prior art as “particular and general” or “narrower and broader”.
The approaches used in both the cases described above not only contradict each other but also, to some extent, do not comply with the requirements for assessment novelty of the invention established by the Russian and Eurasian legislations.
At the same time, the approach used for the Russian patent No. 2488999 is rather close to the approaches of the EAPO used to evaluate novelty of the invention in similar situations.
By contrast, the approach to consideration of the Eurasian patent is quite alarming if the practice develops further in this direction: namely, in recognising inventions as not new based on some general disclosure of a means/subject/method in the prior art.
Such an approach makes it pointless to invest in long-term and expensive projects in studying already known medicines in order to increase their efficacy and safety and in development of new forms of pharmaceutical drugs that are more user-friendly, ie, in creation of inventions the value of which for the public is as high as developing new biologically active substances.










